The Overview Tricentis Leverages Cprime's AWS Expertise to Achieve SOC 2 Compliance About Tricentis Flood:…
Case Study
Transforming a Major Diagnostic Imaging Device Manufacturer with Cprime and SAFe© Agile
Company Details
Industry: Diagnostic Imaging Device Manufacturing
Company Size: 66,000 Employees
Location: United Kingdom, Germany
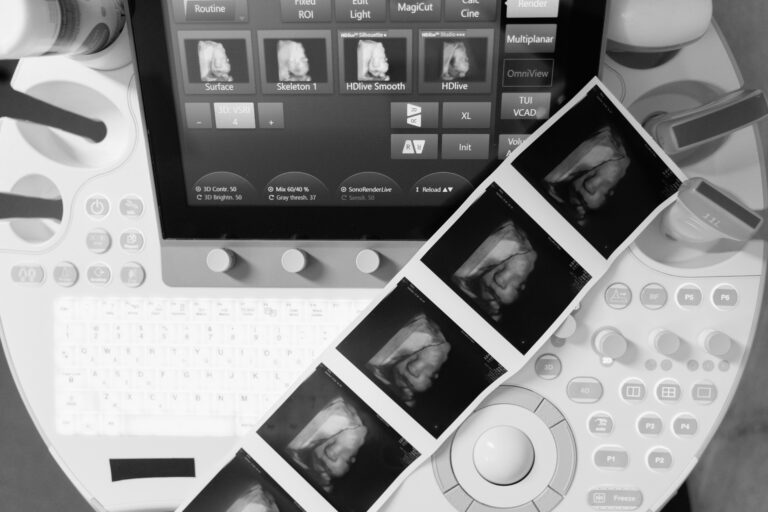
Products: Diagnostic imaging devices — radiography (X-rays), ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI)
Cprime Services:
Executive Summary
With teams on multiple continents struggling to predictably deliver a complex product, this top medical imaging manufacturer turned to Cprime to align its teams with SAFe© agile ways of working. With the help of Cprime trainers, they significantly improved transparency, delivery rates, and employee satisfaction.
Overview
With over 178 years in the field of medical device technology, this Cprime client has been manufacturing medical imaging devices for 125 years — its first commercial diagnostic imaging product was released in 1896, one year after the x-ray tube was invented by Wilhelm Conrad Röntgen.
Since its earliest days, this top-three manufacturer has remained at the forefront of the medtech manufacturing industry; it currently maintains a 23% share of a global market valued at US $40 billion annually. Further, its diagnostic imaging devices are commonplace in hospitals worldwide. These include radiography (X-rays), ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI).
The Challenge
The manufacturer had development partners, manufacturing and R&D facilities spread across Germany, Portugal, the United States, United Kingdom, and Asia. It also had regulatory concerns in every market it occupied. As a result, it wanted to align teams involved in the development of MRI machines across interconnected teams.
“It is an incredibly large organization,” says Stuart Griffith, Cprime SAFe© Program Consultant. “If you look across all of their value delivery trains, suppliers, and researchers the number of people involved in the end-to-end delivery of every MRI product totals in the five-figure range.” By comparison, few companies work at that level of scale and complexity across similar distances.
Creating Consistent, Connected Ways of Working Across Multinational and Multidisciplinary Teams

“You’re only as fast as your slowest runner when you’re building a complex product,” says Sukie Kang, Cprime SPCT & Lean-Agile Transformation Coach. “The manufacturer’s German operations had already adopted a lean agile methodology towards developing and manufacturing the hardware, but one of their key UK development teams was still using traditional ways of working and Gantt charts to remain organized.”
The manufacturer wanted to improve coordination between the European and UK MRI development teams to better integrate with dependent teams. However, there were other issues to consider. Transforming the highly specialized UK team would take more than implementing new processes. The Cprime team had to affect fundamental cultural changes within the manufacturer’s leadership and team members.
“Most people on the team had been there for ten or even twenty years. Over that time some had not evolved their ways of working,” says Griffith. “Because they were technically and academically oriented, they were initially quite resistant to shifting the way they approached problem solving.”
The Solution: Cprime as a SAFe© Agile Gold Partner
Above all, it based the decision on Cprime’s proven track record as SAFe© Agile Gold Partners. Particularly because of their experience in transforming hardware manufacturing and development processes at scale in the UK. Specifically, the Cprime brief was to begin training, coaching, and consulting with the UK team to educate people in lean agile processes, encourage transparency, and reduce delay-to-delivery. The engagement involved helping the UK team transition to a more agile form of product delivery and improving their communication with the European teams and organizational coordination layer.
“Improvements in transparency have resulted in a real understanding of the work to be done and an adjustment in the organization’s expectations. The teams are much more engaged in their work, and our recent Inspect and Adapt event proved that they are solving problems and creating realistic planning horizons.” — Executive Sponsor
“All the MRI teams needed a consistent way of working to effectively integrate and deliver all of these systems together,” says Griffith. “As the only outlier still dependent on traditional processes, the UK team was under pressure to adapt to the common agile framework and use that framework to attain better transparency.” The organization needed a way to understand what was causing delays, how they could correct those delays to speed up product delivery, and, finally, have the confidence and supporting process in place to establish and meet realistic goals.
“They had to start speaking the same language as the rest of the solution train and improve their speed, quality, and predictability,” Kang adds.
Planning for a successful transformation
Using their own agile methodology, the two-person Cprime team began by creating a transformation plan. This involved forming internal teams, ensuring everyone from the UK team leadership was clear on their roles, and establishing all the activities necessary to conduct the first program increment (PI) planning event. It was a planning cycle the UK team would go on to hold independently once every twelve weeks.
“The first cohort of people we trained were the internal change agents. The second cohort is always the leadership — it’s a proven and successful pattern,” says Kang. “Transformations fail if leadership is not trained or engaged in the process.”
So Cprime provided the UK team with intensive coaching in the run-up to the first PI planning session.
“We were very hands-on with coaching and support, helping them uncover and formulate solutions to their challenges,” says Griffith. “We tapered off our involvement as their scrum master, release train engineer (RTE), architects, and team members began communicating effectively and independently solving their issues in later PI iterations.”
Overcoming resistance
“We would run additional sessions using case studies from similar industries demonstrating where their peers had gone down similar paths,” says Kang. “We wanted to make them understand they had the ability to be proactive. Rather than saying ‘It has always been like this,’ they had the power and responsibility to say, ‘This is unacceptable. What can we do to improve it?’”
The Results: The Right Cadence to Meet Organizational Goals
Over the course of nine months encompassing three complete PI planning cycles, the UK teams saw substantial improvements. The changes affected both the manufacturer’s leadership and the UK team’s morale and productivity.
“Improvements in transparency have resulted in real understanding of the work to be done and an adjustment in the organization’s expectations,” says an executive sponsor. “The teams are much more engaged in their work, and our recent Inspect and Adapt event proved that they are solving problems and creating realistic planning horizons.”
The UK team’s delivery rates also improved significantly. Where the team overshot 42% of their deadlines in the first PI session, they reduced that figure to 15% by the end of session two. An internal survey reports greater employee satisfaction and a greater feeling of control over their workload as a result of the Cprime coaching and agile methodology.
In addition to meeting their own goals, the UK had aligned with teams across three continents.
“Everyone was working at the same cadence. The UK had the same heartbeat as its global counterparts,” concludes Kang. “That just made things easier for everybody. It supported the whole organization’s time-to-market goals, its quality goals, and improved its predictability, resulting in a more satisfied customer base.
Interested in similar results for your organization? Explore our flexible group training solutions.
About Cprime
Cprime is an industry-leading, full-service global consulting firm with a focus on providing integrated and innovative solutions around digital transformation, product, cloud, and technology. With over 20 years’ experience, we provide strategic and technical expertise to businesses across more than 50 industries. Our team of advisors and technical experts have the know-how to meet organizations where they are to develop actionable solutions and solve business challenges. We also collaborate with our expansive network of partners to design, deploy, and harmonize technology stacks across organizations. Our mission is to empower visionary business leaders and teams to reimagine the future of work to achieve better outcomes.
Want to share with a colleague? Download the PDFFeatured Team Members

Sukie Kang
Cprime SPCT & Lean-Agile Transformation Coach
Sukie has over 20 years experience across a wide variety of industries. He works with organisations that are undergoing a transformation of ways of working. He has worked with leaders and teams to adopt lean principles and the Agile mindset. Sukie has an MBA from Henley Business School in the UK.

Stuart Griffith
Cprime SAFe© Program Consultant
Stuart has 20 years of experience in the IT industry with a focus on coding and coaching technical teams. He also has experience managing 20+ stakeholders at a time and technical teams based in multiple countries. Stuart's recent accomplishments include program managing a digital innovation lab looking after the entire end-to-end process of green field work for Astrazenica.

